Polycyclic aromatic hydrocarbons PAHs are valuable model systems for both neutral and charged graphene and their chemical derivatives. The water-graphene interaction is of paramount importance in materials chemistry. Theoretical predictions and careful experiments have shown that the graphene wetting characteristics are highly sensitive to small changes in the chemistry on the graphene surface. This implies that applications ranging from battery technology to electrocatalysis depend on interfacial phenomena that contemporary theoretical models only poorly describe. Experimentally probing the molecular level details of the interaction of single-layer graphene with water is also rather challenging, due to pitfalls in the preparation of clean single-layer graphene films and the size and shape heterogeneity of graphenic nanostructures synthesized in typical chemical preparations.
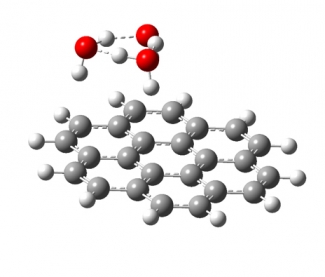
We study the interaction of water molecules with charged PAHs in mass selected cluster ions of the form PAH+/-·(H2O)n using cryogenic infrared photodissociation spectroscopy, and compare the experimental results with theoretical predictions. Multiple water molecules will form hydrogen bonded, networked subclusters on the surface of PAHs (see image below). We are interested on how the shape of the PAH influences the geometry of the water networks formed on its surface, how the charge density in the PAH frame changes the interaction between the water network and the PAH, and how quantum nuclear effects (e.g., tunneling between structural motifs) manifest themselves in the vibrational spectra of these clusters.
This project is a collaboration with the group of Prof. Joel Eaves (CU Chemistry). Our work is funded by the Gas Phase Chemical Physics Program of the Department of Energy (Office of Basic Energy Sciences).