Research Highlights
Biophysics | Other
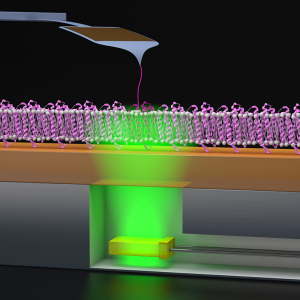
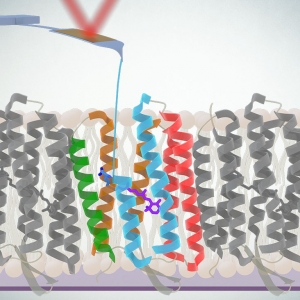
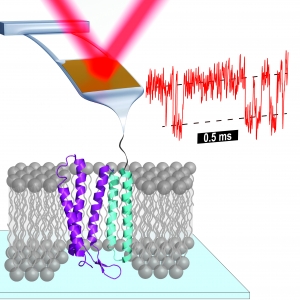
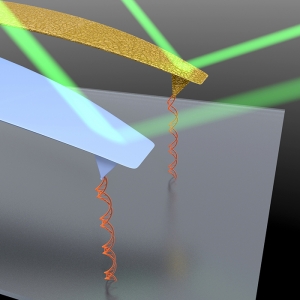
Probing Proton Pumping: New Findings on Protein Folding in bacteriorhodopsin (bR)
Published: February 05, 2024
PI: Thomas Perkins
Biophysics
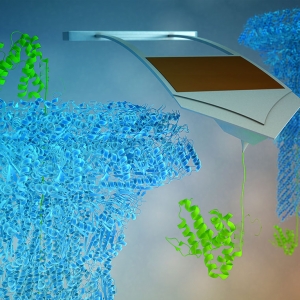
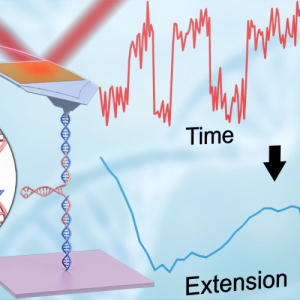
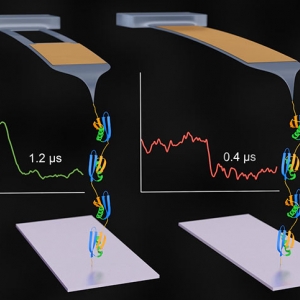
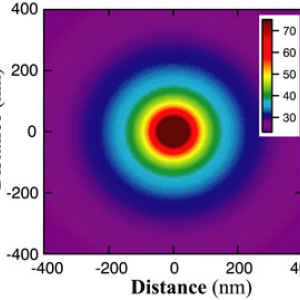
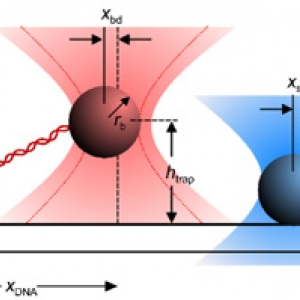
The Forces involved in Folding Proteins
Published: March 22, 2021
PI: Thomas Perkins
Biophysics | Precision Measurement
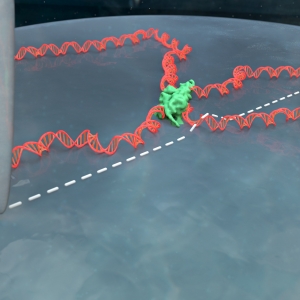
DNA imaging, ready in five minutes
Published: July 16, 2019
PI: Thomas Perkins
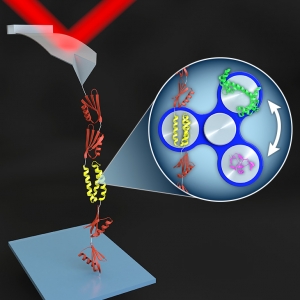
Nanoscience
The Land of Enhancement: AFM Spectroscopy
Published: October 16, 2015
PI: Thomas Perkins
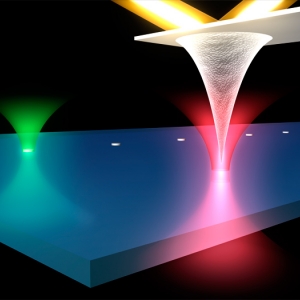
Biophysics | Nanoscience
bR Phone Home
Published: February 04, 2014
PI: Markus Raschke | PI: Thomas Perkins
Biophysics | Nanoscience
How to Marry a Microscope
Published: April 10, 2009
PI: Thomas Perkins
Biophysics | Nanoscience
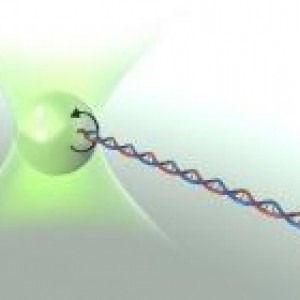
DNA: Force of Nature
Published: February 07, 2008
PI: Thomas Perkins
Biophysics | Nanoscience
Sightseeing along a DNA Strand
Published: May 01, 2005
PI: Thomas Perkins