Understanding chemistry requires understanding both molecules and quantum physics. The former defines the start and end of chemical reactions, the latter dictates the dynamics in between.
JILA researchers now have a better understanding of both.
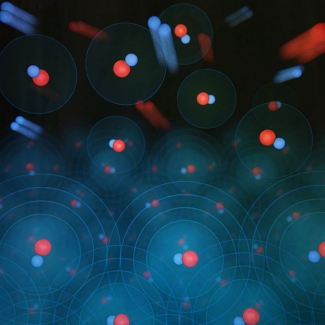
For the last decade, JILA researchers have expertly chilled, and then combined, atoms into ultracold polar molecules. But today, researchers in the Ye group announced achievement of the next step: combining cold atoms into quantum degenerate polar molecules. This new form of molecular matter amplifies many-body quantum effects between molecules and has already furthered our understanding of chemistry at the quantum level.
Calm Cool Down
Studying molecules is not easy. At room temperature, they rotate, vibrate, and run around—and into each other—like caffeinated school children. But researchers can create calm molecules, and therefore easier-to-study molecules, through cooling.
In 2008, JILA Fellows Deborah Jin and Jun Ye created the first gas of ultracold polar molecules by cooling and combining two ultracold atoms. At 500 million times colder than the average temperature of Earth, these polar molecules no longer rotate or vibrate, but they can collide—if researchers permit it.
Obtaining this level of control for any molecule is fascinating, but it is particularly so for polar molecules. Polar molecules, such as H2O, have an electric charge difference across either end. This small charge difference manifests into large-scale physical properties, such as solubility, melting and boiling points, and surface tension.
But this control, however fascinating, was only surface deep. While Jin and Ye could control how the molecules interacted with their nearest neighbor, they could not control how a molecule might interact with farther neighbors. To achieve that control, they’d need to create degenerate polar molecules.
Cold, Ultracold… Degenerate?
Quantum degeneracy is not simply colder than ultracold. With degeneracy, it’s no longer temperature that is important, but space, or lack thereof.
For a gas of molecules to be degenerate, the “size” of each molecule must be larger than the distance between nearest neighbors. This means that the molecules overlap, thereby creating a big pile of indistinguishability where it is impossible to tell where one molecule ends and another begins.
There are two ways to make molecules degenerate. The first is to squeeze molecules together so hard they are forced to overlap, which is what happens in very dense places, like the center of neutron stars. The other way is to cool the molecules, thereby expanding their quantum size (defined by the de Broglie wavelength) .
JILA researchers opted for the cold route. Specifically, our researchers opted to cool their polar molecules down to their “ground-state,” or as cold as physics permits. This decision meant nearly a decade of developing laser and atom trapping technologies and years of fine-tuning ratios of temperature and atom quantities, but according to Luigi De Marco, the lead postdoc of this research, these challenges did not hamper ambition.
“Pushing molecules into this regime of quantum degeneracy is something that people have talked about doing with ultracold [polar] molecules since they were first made ten years ago,” said De Marco with an eager grin.
To make their degenerate molecules, De Marco and his team cooled rubidium and potassium atoms to degeneracy and then combined them. This process was similar to the process to create ultracold molecules, but much colder.
An early hurdle that the group had to jump was the number of cold rubidium atoms.
“In our experiment, Rubidium atoms do all the cooling for potassium atoms,” said De Marco. “So, if you don’t have enough rubidium atoms, and keep trying to make it colder and colder, eventually your potassium is going to stop getting colder, and you are going to lose all your rubidium.”
The rubidium essentially acts as ice cubes for the potassium, explained De Marco. “If you have a hot drink and not enough ice cubes to cool it down, you’re going to end up with no ice cubes and still a hot drink.”
Degeneracy Brings About Quantum Surprises
Upon creating their ground-state polar molecules, the team observed a stark chemical change.
Within ultracold molecules, the group had long observed an uncontrollable chemical reaction that slowly destroyed their molecules, resulting in loss.
“What we measured in the past was that if we made the molecules cooler, the loss rate linearly dropped, and if we made them hotter, it linearly increased,” said De Marco.
But when the molecules became degenerate, De Marco and his team saw a surprising change.
“As we brought the temperature lower and lower, further and further into the degenerate regime, the suppression of chemical reactions was more than linear,” said De Marco. In fact, they were able to lower the reaction rate by a factor of four times than would be possible when not degenerate.
“And we confirmed that is not an effect of just the temperature itself,” De Marco added. Because degeneracy depends on overlap, not just temperature, the team was able to create nondegenerate clouds of the same temperature by using fewer molecules. Without the degeneracy, the chemical reaction rate remained linear.
“it was really surprising that the degenerate regime allowed us to beat this linear scaling and suppress these reactions.”
According to De Marco, the molecule reaction is suppressed by quantum effects that are enhanced in the degenerate regime. Whereas classical molecules interact with only nearest neighbors, quantum molecules are influenced by all surrounding molecules. These influences can prevent reactions because of certain quantum exclusion rules.
“We see that chemical reactions turn off as the gas becomes more and more degenerate. That was exciting for us, as no one has ever seen that sort of direct interplay between quantum correlations and chemistry.”
De Marco and his team have not only created the first degenerate gas of molecules, but demonstrated its powerful ability to enhance quantum effects. With further study of gases like this, De Marco says that quantum computers using molecular chemistry for data storage and more precise measurements of our universes’ fundamental symmetries may be possible.
This research was published online today (17 January 2019) in Science Express. In addition to Luigi De Marco, additional authors include JILA Postdoc Giacomo Valtolina, JILA graduate students Kyle Matsuda and William G. Tobias, recent JILA graduate Jacob P. Covey, and JILA Fellow Jun Ye. JILA Fellow Deborah Jin, who began collaborating with Ye on this experiment over a decade ago, passed away in 2016.
Written by Catherine Klauss