X-rays are notorious for damaging molecules, including those in our bodies. High in the upper atmosphere, X-rays from the Sun break apart simple molecules like nitrogen (N2) and drive chemical reactions affecting the Earth. For these reasons, it’s important to understand exactly how radiation interacts with, damages, or destroys specific chemicals.
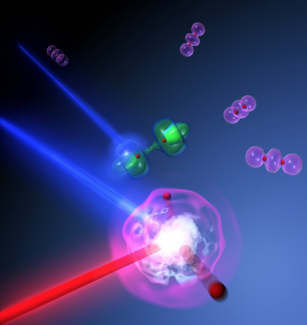
Recently, Fellows Margaret Murnane and Henry Kapteyn worked with Graduate Student Etienne Gagnon, Senior Research Associate Arvinder Sandhu, and colleagues from Kansas State University and the University of Tsukuba (Japan) to capture the sequence of events that occur as a molecule explodes after being irradiated by X-rays. First, they excited the molecules with ultrafast (5 femtosecond) flashes of X-ray light that eject an electron from a molecule (in this case N2), leaving the molecule in a super-excited state that eventually causes it to break apart. Because the X-ray pulses were faster than the time it takes the N atoms in the molecule to separate, the scientists could then use a fast laser pulse to peer inside the super-excited molecule as it separated into pairs of nitrogen atoms and nitrogen ions. In the process, they discovered exactly how X-rays can cause the N2molecule to explode!
As the scientists already knew, X-rays can knock an electron out of an N2 molecule. What they discovered, however, was that the molecule could fall apart in more than one way, depending on which electron was ejected. If a deeply bound (inner-shell) electron was ejected, the disturbance in the electron cloud surrounding the two positively charged atomic nuclei was strong enough to fragment the molecule. However, if a less deeply bound (outer-shell) electron was dislodged, there was enough left-over energy to “shake up” a second electron and simultaneously thrust it into high orbit far (4 Å) from the molecule’s center. At this distance, the electron was too far away to help shield the positively charged atomic nuclei from each other, causing the N2 molecule to violently explode.
With this experiment, Kapteyn and Murnane have taken the first step into a whole new area of radiation-based chemical physics. In the future, they plan to explore complex electron dynamics and highly excited states in a range of atomic, molecular, and materials systems. - Julie Phillips