A solvent is something that dissolves or disperses something else. It's the water in salt water, the alcohol in cough syrup, the lactates or ethers in inks. For many of us, solvents are the background music of the chemistry taking place all around us. But this isn't how Fellow Carl Lineberger and his colleagues in chemical physics think about solvents. Lineberger, Former Research Associate Vladimir Dribinski, Graduate Students Jack Barbera and Josh Martin, and student visitor Annette Svendsen see them as key players in some chemical reactions, right down to the level of quantum mechanical interactions.
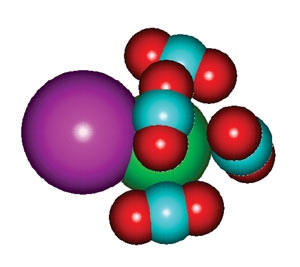
With the help of theorist Robert Parson and his graduate student, Matt Thompson, the researchers have shown just how important solvents can be in determining the rate of one reaction: the recombination of iodine-bromide clusters [IBr-(CO2)n] dissociated by laser light. The [IBr-(CO2)n] cluster shown at right consists of an iodine atom (purple), a negatively charged bromine atom (green) and four carbon dioxide solvent molecules. In the latter, the oxygen atoms are red and the carbon turquoise.
When the Lineberger group set out to study the dynamics of solvent-induced recombination of [IBr-(CO2)n] clusters, they thought they knew what to expect. After all, they'd done similar experiments on an ion of molecular iodine (I2-) dispersed in carbon dioxide [I2-(CO2)n]. They'd even figured out how to add solvent molecules to the clusters one at a time, which allowed them to monitor changes occurring with increasing solvation. In the I2-(CO2)n system, the iodine molecules become surrounded by a solvent shell that builds randomly from wherever the first CO2molecule aligns itself. When laser light dissociates the iodine molecule into two iodine atoms, the atoms stay inside the shell and rapidly recombine. The group found that even when a small number of solvent molecules are present, the iodine atoms recombine in about 10 ps. The rate of recombination increases as more solvent molecules are added.
With [IBr-(CO2)n] clusters, the researchers found that the solvent shell always forms first around the bromide end, probably because it is smaller than the iodine. However, when they photodissociate the IBr- molecule, things get very interesting. In clusters with 6 or fewer solvent molecules, the iodine and bromine atoms recombine in about 10 ps, as in the I2-(CO2)n experiments. However, the recombination rate shoots up to and stays at 1000 ps for clusters with 8-10 solvent molecules. The addition of more solvent molecules rapidly brings the recombination rate back in line with the I2-(CO2)n experiments.
When you get an experimental result this unexpected, the best thing to do is to consult a theorist, according to Lineberger. Thompson and Parson were able to show that in clusters of 8-10 solvent molecules, the CO2 molecules are actually trapping IBr- in its dissociated state. In other words, asymmetric solvation of IBr- has such a dramatic effect on the formation of the solvent shell that it causes excited-state trapping, i.e., it alters the quantum state of the solute. Since the addition of more solvent breaks down the trap, the phenomenon only occurs over a small range of solvent sizes. Interestingly, even though it takes a long time, the IBr- molecules eventually do get back together, even when they're surrounded by 8-10 solvent molecules.
Lineberger says a combination of theory with experiment is responsible for the leap in understanding of asymmetric solvation, adding that "without quantum mechanics, we'd still be scratching our heads." - Julie Phillips
The findings presented here will appear in the November 1, 2006, issue of The Journal of Chemical Physics.