"Chemistry is a highly improbable science," says Graduate Student Mike Deskevich, who adds "It's good for life on Earth that things are so unreactive." For instance, if chemical reactions happened easily and often, oxygen in the air would cause clothing and other flammable materials to burst into flame. In addition to making life difficult, high probability chemistry would render theoretical chemical physics much less interesting. As it is, theorists spend months determining the particular molecular shapes, vibrations, and energy states that make the simplest chemical reactions possible.
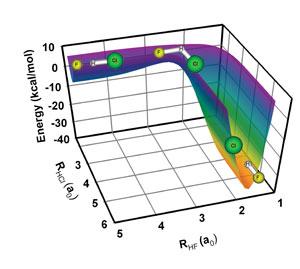
Recently Deskevich worked on a tricky theoretical problem with Fellow David Nesbitt; Michael Hayes, a graduate student in chemistry; Kaito Takahashi, a postdoc in Taiwan; and Professor Rex Skodje in CU's Department of Chemistry and Biochemistry. The researchers created the "potential energy surface," or PES (right), which shows in theory how the potential energy of a reaction changes with the position of the reactants and products. A PES contains thousands of points created via a detailed quantum mechanical analysis of the energy states and atomic and molecular orientations that play a role in the reaction. The process of creating PES takes hundreds of hours, but it's time well spent. How a molecule travels on a good PES reflects its real-life behavior.
The researchers used the new PES to monitor the transfer of a hydrogen atom from a chlorine atom to a fluorine atom, which is a very simple chemical reaction. In chemistry shorthand, the reaction looks like this: HCl + F → HF + Cl. What they found was that it doesn't take a lot of energy to make the reaction happen if the reactants collide in a very specific orientation. Energetically, the reactants have to climb up a hill and go around a corner for a reaction to take place. For that to happen, the fluorine atom must collide with the hydrogen end of the HCl molecule such that the two reactants form a transitory F-H-Cl molecule, which is bent at the H, forming an angle of exactly 123.5°.
To form the perfect angle, the F has to hit the HCl at a slightly wider angle because the collision itself causes the transition state to bend further into the right conformation. If the F collides straight on, there is only enough energy to get up the hill, but not around the corner. So no reaction happens. If the F collides at an angle of 123.5° or less, the transition state is too bent to even get up the hill. What's remarkable about this chemical reaction is that if even one F hits the bull's eye, the reaction releases enough energy to help the remaining reactants get up the hill and around the corner. If this reaction is uncontrolled, it can even explode. - Julie Phillips
The work reported here appeared in the June 14, 2006, issue of The Journal of Physical Chemistry.