A solid understanding of the structure and behavior of atoms is important for understanding the physical world, from the basic building blocks of nature to the inner workings of modern technology. However, education researchers have expressed different opinions regarding the best way to teach students the ins and outs of atoms. In particular, some have even recommended doing away with teaching the older and simpler Bohr model, asserting that it inhibits students’ ability to understand the quantum nature of electrons in atoms. This claim recently prompted research associate Sam McKagan and her colleagues, CU Assistant Professor Kathy Perkins and Fellow Carl Wieman, to not only test this claim, but also to develop curriculum on models of the atom, including the models proposed by Niels Bohr in 1913 and Erwin Schrödinger in 1926.
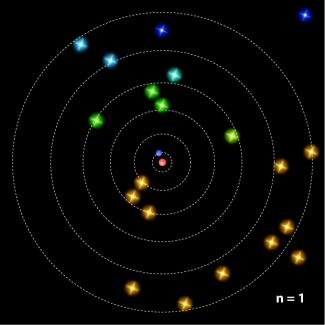
The researchers found that teaching the Bohr model, which describes electrons as point particles orbiting the nucleus at fixed distances, did not prevent CU physics students from grasping the more complex Schrödinger model. The latter model views electrons as clouds of probability. The key was using the Bohr model in a curriculum designed to develop students’ skills in scientific model building. If the Bohr model was presented in an historical context with ample opportunity to compare and contrast it with other models of the atom, students were more likely to eventually adopt Schrödinger’s view of the atom than if the models were presented in isolation, simply as different sets of equations and rules.
Indeed, CU students found it much more difficult to understand the reasons why new models were necessary than they did to grasp the key features of the different models. For this reason, McKagan and her colleagues made explicit connections between the models for students and presented the historical context in which they were developed. In this way, the students were introduced to a critical skill for furthering scientific progress — model building — as they learned about atomic structure.
To help students effectively visualize different models of the atom, McKagan and her colleagues have developed new interactive computer simulations designed to help students effectively build and evaluate models of the atom. The new simulations, called Models of the Hydrogen Atom and Rutherford Scattering, are available on the Physics Education Technology website (http://phet.colorado.edu/new/index.php). - Julie Phillips