Research Highlights
Displaying 421 - 440 of 470
Atomic & Molecular Physics
Universal Attractions
PI(s):
Deborah Jin
Laser Physics
Team Photon
PI(s):
Henry Kapteyn | Margaret Murnane
Astrophysics
Planetary Shakeup
PI(s):
Phil Armitage
Astrophysics
In Soot I Sleep
PI(s):
Jeffrey Linsky
Chemical Physics
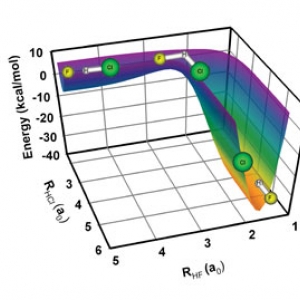
Bull's Eye!
PI(s):
David Nesbitt
Precision Measurement
Wanted: Gravitational Waves
PI(s):
Peter Bender
Chemical Physics
Spectral Shapes
PI(s):
David Nesbitt
Chemical Physics
Trapped!
PI(s):
W. Carl Lineberger
Laser Physics
Magic Light
PI(s):
Jun Ye
Biophysics | Nanoscience
Gold Fever
PI(s):
Thomas Perkins
Atomic & Molecular Physics | Nanoscience
Constant Vigilance
PI(s):
Heather Lewandowski
Atomic & Molecular Physics
Partnership in Time
PI(s):
Jun Ye
Astrophysics
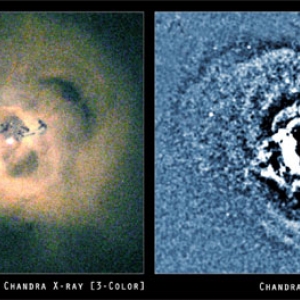
Bubbling Clusters of Galaxies
PI(s):
Mitch Begelman
Atomic & Molecular Physics
Flashdance!
PI(s):
Chris Greene
Astrophysics
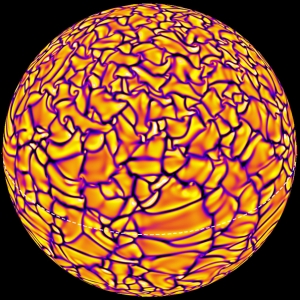
As the Sun Turns
PI(s):
Juri Toomre
Biophysics | Chemical Physics
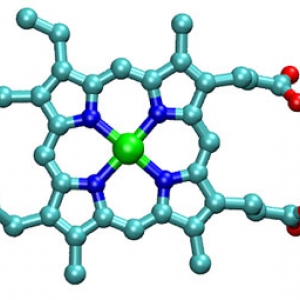
Heme Motions
PI(s):
Ralph Jimenez
Laser Physics
Molecular Fingerprinting
PI(s):
Jun Ye
Astrophysics
Flare Up!
PI(s):
Phil Armitage | Rosalba Perna
Astrophysics
Bubble Shock Trains
PI(s):
Mitch Begelman
Atomic & Molecular Physics
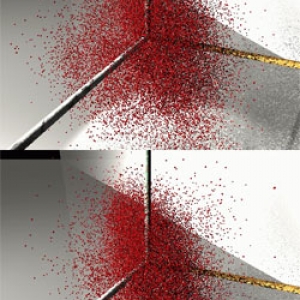
Body of Evidence
PI(s):
Chris Greene